The GBS|CIDP Foundation International is committed to supporting research happening for GBS, CIDP, and variants such as MMN. Sometimes, that research is done by doctors at academic institutions. Other times, it is done by researchers and doctors working for biotechnology, engineering, or pharmaceutical companies. The Foundation also believes that any research being done for GBS, CIDP, or variants such as MMN should include the perspective of patients living with that condition. By clicking one of the links below, you will find opportunities to be involved in the research process.
Research & Clinical Trials
Read on to learn more about the basics of clinical research & why it is important to participate. Alternatively, click here to learn more about the ongoing clinical trials currently recruiting participants.
The Research Process: Bench to Bedside
The practice of medicine and treating patients involves a great deal of work “behind the scenes”. Many doctors, biologists, chemists, pharmacists, and other researchers dedicate their ideas, knowledge, and expertise to study potential new treatment options to treat, and possibly cure, medical conditions. This usually takes years, sometimes decades, and plenty of funding. Before a potential new treatment moves into the clinical trial phase, where it is given to human patients, the safety profile of the potential new treatment is studied extensively in the lab and often tested in animal models. This way, patients that take part in clinical trials can be assured that only the best, and most safe, potential new treatments make it to the clinical trial phase.
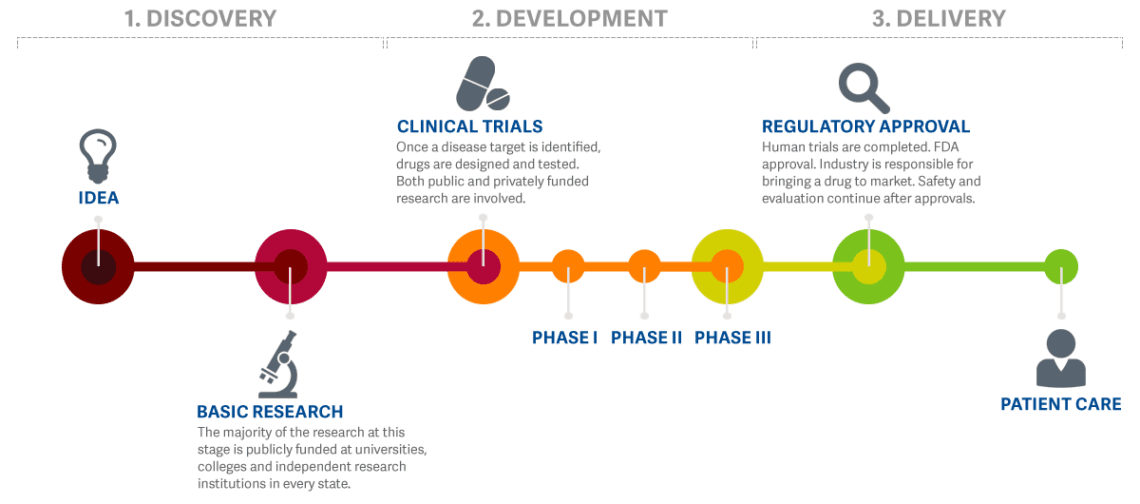
Before Clinical Trials in humans can start, investigators must submit all the data generated in the laboratory and in animals, for review by the scientists at FDA, to see whether there is any indication that this treatment might potentially be harmful. Investigators must also share some manufacturing details and characterization of the new chemical entity proposed to be tested in humans. The FDA must either request additional information from the investigator within 30 days or allow the clinical trials to begin.

