By Lisa Butler
In 2020 as the Foundation worked with the FDA, for the first time, what I feel is most notable – and praiseworthy – is their straightforward adherence to science, and their tremendous efforts to bring the patient perspective into the decision-making process. I feel even more confident in the leadership of the FDA with each and every correspondence. And as a parent of a GBS patient myself, and the Executive Director of the GBS|CIDP Foundation International, having confidence in our federal agencies, that they not only understand these rare neurologic conditions to the fullest extent but that they are truly listening to our stories and the specific concerns of our community, is paramount.
Our first meeting with the FDA was on September 29, 2020, when the Foundation by invitation of the FDA, organized a patient-led listening session on Guillain-Barre Syndrome. More than 30 members of various branches within the FDA logged on to the virtual meeting to hear about patients’ experiences with GBS. The FDA’s role was to listen and learn about what life is like during and after a GBS diagnosis. The 6 speakers, including patients and caregivers from our community, captured various aspects of life with GBS, including:
- Treatment & Diagnosis When the System Works
- Treatment & Diagnosis When the System Does NOT Work
- The Emotional Burden on the Family
- Rehab, Recovery, and Residuals
- Variant Perspective
The Foundation also was given the opportunity to describe the common unmet needs of the community and express a desire to work more closely with the FDA in the future. A full summary of the meeting can be found here.
As a follow up to the initial listening session, the Foundation was invited by the FDA’s Center for Biologics Evaluation and Research’s (CBER) to participate in an open session to discuss Emergency Use Authorization of the Moderna, Inc., COVID-19 vaccine for the prevention of COVID-19 in individuals 18 years and older. The meeting took place on December 17, and on behalf of the Foundation, I participated and represented the voice of the GBS|CIDP Foundation. Our key message, was to inform the agency that while we are in a public health emergency, and a safe and effective vaccine against COVID-19 serves as a beacon of hope for many Americans, the Guillain-Barre Syndrome community feels a renewed sense of worry. I noted that though the data is still quite limited, the Foundation’s Global Medical Advisory Board feels hopeful that the relative risk of GBS after a COVID-19 vaccine is comparable to the risk of the flu vaccination, very small or nonexistent.
The below is a summary of the current community inquires and issues with regard to the COVID-19 inquiries, and encapsulates our presentation in the Dec. 17 meeting.
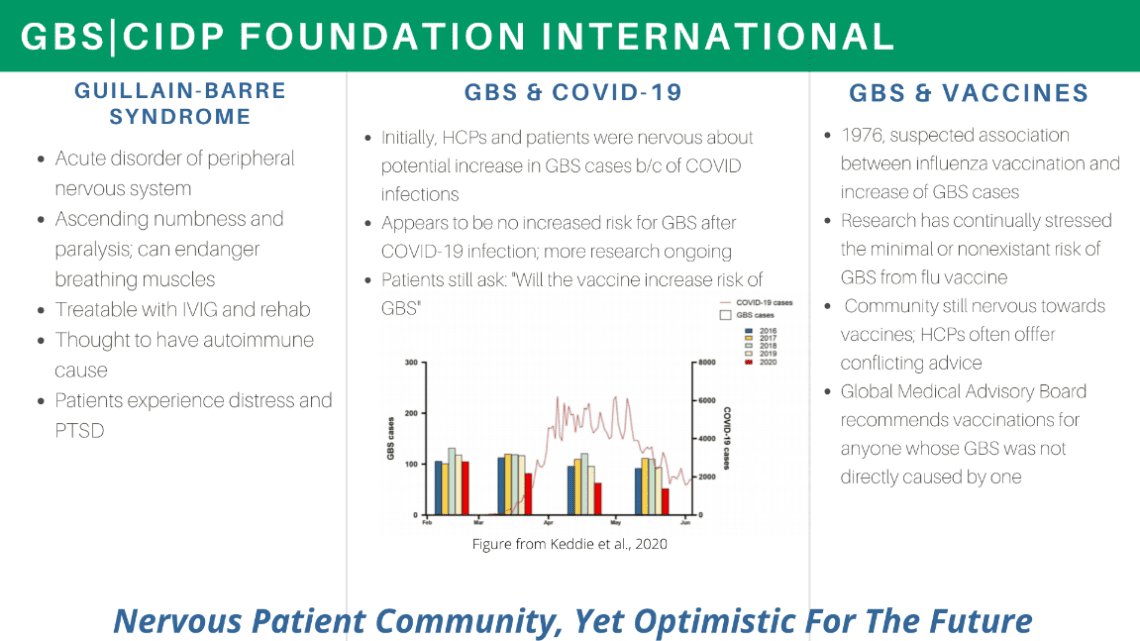
Our goal was to encourage the FDA to provide as much information as possible to assure GBS patients and the broader community that they are being given a vaccine that is as safe as it is effective. The Meeting was open to the public, and can be viewed below. I spoke on behalf of the Foundation during the public comments section, for a brief period, within the hour of 12-1pm EST.
More information can be found at: https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-december-17-2020-meeting-announcement
I look forward to sharing a summary of meeting outcome in upcoming weeks. As well, through 2021 and beyond, we look forward to representing the voice of our patients, and sharing information regarding our continued relationship with the FDA.
